Apraclonidine Prescribing Information
Package insert / product label
Generic name: apraclonidine hydrochloride
Dosage form: ophthalmic solution
Drug class: Ophthalmic glaucoma agents
Medically reviewed by Drugs.com. Last updated on Jul 6, 2022.
On This Page
Apraclonidine Description
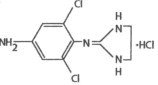
Apraclonidine Ophthalmic Solution, USP 0.5% as base contains apraclonidine hydrochloride, an alpha adrenergic agonist, in a sterile isotonic solution for topical application to the eye. Apraclonidine hydrochloride is a white to off-white powder and is highly soluble in water. Its chemical name is 2-[(4-amino-2,6 dichlorophenyl) imino]imidazolidine monohydrochloride with an empirical formula of C9H11Cl3N4 and a molecular weight of 281.57. The chemical structure of apraclonidine hydrochloride is:
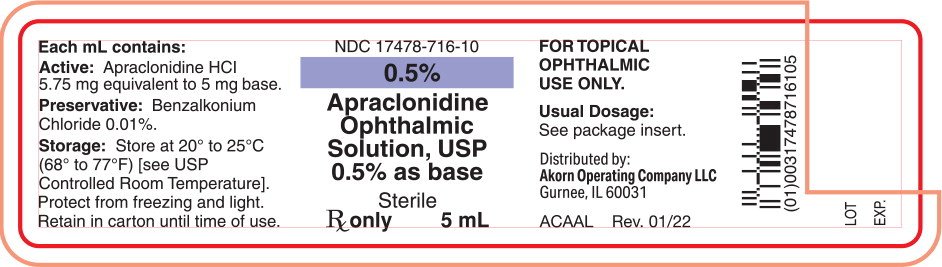
Each mL of Apraclonidine Ophthalmic Solution, USP 0.5% as base contains:
Active: apraclonidine hydrochloride 5.75 mg equivalent to apraclonidine base 5 mg;
Inactives: sodium acetate, sodium chloride, sodium hydroxide and/or hydrochloric acid may be added to adjust pH (4.4 to 7.8) and water for injection;
Preservative: benzalkonium chloride 0.01%.
Apraclonidine - Clinical Pharmacology
Apraclonidine hydrochloride is a relatively selective alpha-2-adrenergic agonist. When instilled in the eye, apraclonidine ophthalmic solution has the action of reducing elevated, as well as normal, intraocular pressure (IOP), whether or not accompanied by glaucoma. Ophthalmic apraclonidine has minimal effect on cardiovascular parameters.
Elevated IOP presents a major risk factor in glaucomatous field loss. The higher the level of IOP, the greater the likelihood of optic nerve damage and visual field loss. Apraclonidine ophthalmic solution has the action of reducing IOP. The onset of action of apraclonidine can usually be noted within one hour, and maximum IOP reduction occurs about three hours after instillation. Aqueous fluorophotometry studies demonstrate that apraclonidine's predominant mechanism of action is reduction of aqueous flow via stimulation of the alpha-adrenergic system.
Repeated dose-response and comparative studies (0.125% to 1.0% apraclonidine) demonstrate that 0.5% apraclonidine is at the top of the dose/response IOP reduction curve.
The clinical utility of apraclonidine ophthalmic solution is most apparent for those glaucoma patients on maximally tolerated medical therapy. Patients on maximally tolerated medical therapy with uncontrolled IOP and scheduled to undergo laser trabeculoplasty or trabeculectomy surgery were enrolled into a double-masked, placebo-controlled, multi-center clinical trial to determine if apraclonidine ophthalmic solution, dosed three times daily, could delay the need for surgery for up to three months.
All patients enrolled into this trial had advanced glaucoma and were undergoing maximally tolerated medical therapy, i.e., patients were using combinations of a topical beta blocker, sympathomimetics, parasympathomimetics and oral carbonic anhydrase inhibitors. Patients were considered to be treatment failures in this study if, in the opinion of the investigators, their IOP was uncontrolled by the masked study medication or there was evidence of further optic nerve damage or visual field loss, and surgery was indicated. Of 171 patients receiving masked medication, 84 were treated with apraclonidine ophthalmic solution and 87 were treated with placebo (apraclonidine vehicle).
Apraclonidine treatment resulted in a significantly greater percentage of treatment successes compared to patients treated with placebo. In this placebo-controlled maximum therapy trial, 14.3% of patients treated with apraclonidine ophthalmic solution were discontinued due to adverse events, primarily allergic-like reactions (12.9%).
The IOP lowering efficacy of apraclonidine ophthalmic solution diminishes over time in some patients. This loss of effect, or tachyphylaxis, appears to be an individual occurrence with a variable time of onset and should be closely monitored.
An unpredictable decrease of IOP control in some patients and incidence of ocular allergic responses and systemic side effects may limit the utility of apraclonidine ophthalmic solution. However, patients on maximally tolerated medical therapy may still benefit from the additional IOP reduction provided by the short-term use of apraclonidine ophthalmic solution.
Topical use of apraclonidine ophthalmic solution leads to systemic absorption. Studies of apraclonidine ophthalmic solution dosed one drop three times a day in both eyes for 10 days, in normal volunteers, yielded mean peak and trough concentrations of 0.9 ng/mL and 0.5 ng/mL, respectively. The half-life of apraclonidine ophthalmic solution was calculated to be 8 hours.
Apraclonidine ophthalmic solution, because of its alpha adrenergic activity, is a vasoconstrictor. Single dose ocular blood flow studies in monkeys, using the microsphere technique, demonstrated a reduced blood flow for the anterior segment; however, no reduction in blood flow was observed in the posterior segment of the eye after a topical dose of apraclonidine ophthalmic solution. Ocular blood flow studies have not been conducted in humans.
Indications and Usage for Apraclonidine
Apraclonidine Ophthalmic Solution, USP 0.5% as base is indicated for short-term adjunctive therapy in patients on maximally tolerated medical therapy who require additional IOP reduction. Patients on maximally tolerated medical therapy who are treated with Apraclonidine Ophthalmic Solution, USP 0.5% as base to delay surgery should have frequent follow-up examinations and treatment should be discontinued if the intraocular pressure rises significantly.
The addition of Apraclonidine Ophthalmic Solution, USP 0.5% as base to patients already using two aqueous suppressing drugs (i.e., beta-blocker plus carbonic anhydrase inhibitor) as part of their maximally tolerated medical therapy may not provide additional benefit. This is because Apraclonidine Ophthalmic Solution, USP 0.5% as base is an aqueous suppressing drug and the addition of a third aqueous suppressant may not significantly reduce IOP.
The IOP lowering efficacy of Apraclonidine Ophthalmic Solution, USP 0.5% as base diminishes over time in some patients. This loss of effect, or tachyphylaxis, appears to be an individual occurrence with a variable time of onset and should be closely monitored. The benefit for most patients is less than one month.
Contraindications
Apraclonidine ophthalmic solution is contraindicated in patients with hypersensitivity to apraclonidine or any other component of this medication, as well as systemic clonidine. It is also contraindicated in patients receiving monoamine oxidase inhibitors (MAO inhibitors).
Precautions
General
Glaucoma patients on maximally tolerated medical therapy who are treated with apraclonidine ophthalmic solution to delay surgery should have their visual fields monitored periodically.
Although the topical use of apraclonidine ophthalmic solution has not been studied in renal failure patients, structurally related clonidine undergoes a significant increase in half -life in patients with severe renal impairment. Close monitoring of cardiovascular parameters in patients with impaired renal function is advised if they are candidates for topical apraclonidine therapy. Close monitoring of cardiovascular parameters in patients with impaired liver function is also advised as the systemic dosage form of clonidine is partly metabolized in the liver.
While the topical administration of apraclonidine ophthalmic solution had minimal effect on heart rate or blood pressure in clinical studies evaluating glaucoma patients, the preclinical pharmacology profile of this drug suggests that caution should be observed in treating patients with severe, uncontrolled cardiovascular disease, including hypertension. The possibility of a vasovagal attack should be considered and caution should be exercised in patients with a history of such episodes.
Apraclonidine ophthalmic solution should be used with caution in patients with coronary insufficiency, recent myocardial infarction, cerebrovascular disease, chronic renal failure, Raynaud's disease, or thromboangiitis obliterans. Caution and monitoring of depressed patients are advised since apraclonidine has been infrequently associated with depression.
Apraclonidine can cause dizziness and somnolence. Patients who engage in hazardous activities requiring mental alertness should be warned of the potential for a decrease in mental alertness while using apraclonidine.
Topical ocular administration of two drops of 0.5, 1.0 and 1.5% apraclonidine ophthalmic solution to New Zealand albino rabbits three times daily for one month resulted in sporadic and transient instances of minimal corneal edema in the 1.5% group only; no histopathological changes were noted in those eyes.
Use of apraclonidine ophthalmic solution can lead to an allergic-like reaction characterized wholly or in part by the symptoms of hyperemia, pruritus, discomfort, tearing, foreign body sensation, and edema of the lids and conjunctiva. If ocular allergic-like symptoms occur, apraclonidine ophthalmic solution therapy should be discontinued.
Information for Patients
Do not touch dropper tip to any surface as this may contaminate the contents.
The preservative in apraclonidine ophthalmic solution, benzalkonium chloride, may be absorbed by soft contact lenses. Contact lenses should be removed during instillation of apraclonidine ophthalmic solution but may be reinserted 15 minutes after instillation.
Drug Interactions
Apraclonidine should not be used in patients receiving MAO inhibitors (see CONTRAINDICATIONS). Although no specific drug interactions with topical glaucoma drugs or systemic medications were identified in clinical studies of apraclonidine ophthalmic solution, the possibility of an additive or potentiating effect with CNS depressants (alcohol, barbiturates, opiates, sedatives, anesthetics) should be considered. Tricyclic antidepressants have been reported to blunt the hypotensive effect of systemic clonidine. It is not known whether the concurrent use of these agents with apraclonidine can lead to a reduction in IOP lowering effect. No data on the level of circulating catecholamines after apraclonidine withdrawal are available. Caution, however, is advised in patients taking tricyclic antidepressants which can affect the metabolism and uptake of circulating amines.
An additive hypotensive effect has been reported with the combination of systemic clonidine and neuroleptic therapy. Systemic clonidine may inhibit the production of catecholamines in response to insulin-induced hypoglycemia and mask the signs and symptoms of hypoglycemia.
Since apraclonidine may reduce pulse and blood pressure, caution in using drugs such as beta-blockers (ophthalmic and systemic), antihypertensives, and cardiac glycosides is advised. Patients using cardiovascular drugs concurrently with apraclonidine ophthalmic solution should have pulse and blood pressure frequently monitored. Caution should be exercised with simultaneous use of clonidine and other similar pharmacologic agents.
Carcinogenesis, Mutagenesis, Impairment of Fertility
No significant change in tumor incidence or type was observed following two years of oral administration of apraclonidine HCl to rats and mice at dosages of 1.0 and 0.6 mg/kg, up to 20 and 12 times, respectively, the maximum dose recommended for human topical ocular use.
Apraclonidine HCl was not mutagenic in a series of in vitro mutagenicity tests, including the Ames test, a mouse lymphoma forward mutation assay, a chromosome aberration assay in cultured Chinese hamster ovary (CHO) cells, a sister chromatid exchange assay in CHO cells, and a cell transformation assay. An in vivo mouse micronucleus assay conducted with apraclonidine HCl also provided no evidence of mutagenicity. Reproduction and fertility studies in rats showed no adverse effect on male or female fertility at a dose of 0.5 mg/kg (5 to 10 times the maximum recommended human dose).
Pregnancy
Apraclonidine HCl has been shown to have an embryocidal effect in rabbits when given in an oral dose of 3.0 mg/kg (60 times the maximum recommended human dose). Dose related maternal toxicity was observed in pregnant rats at 0.3 mg/kg (6 times the maximum recommended human dose). There are no adequate and well controlled studies in pregnant women. Apraclonidine ophthalmic solution should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Adverse Reactions/Side Effects
In clinical studies the overall discontinuation rate related to apraclonidine ophthalmic solution was 15%. The most commonly reported events leading to discontinuation included (in decreasing order of frequency) hyperemia, pruritus, tearing, discomfort, lid edema, dry mouth, and foreign body sensation.
The following adverse reactions (incidences) were reported in clinical studies of apraclonidine ophthalmic solution as being possibly, probably, or definitely related to therapy:
Ocular
The following adverse reactions were reported in 5 to 15% of the patients: discomfort, hyperemia, and pruritus.
The following adverse reactions were reported in 1 to 5% of the patients: blanching, blurred vision, conjunctivitis, discharge, dry eye, foreign body sensation, lid edema, and tearing.
The following adverse reactions were reported in less than 1% of the patients: abnormal vision, blepharitis, blepharoconjunctivitis, conjunctival edema, conjunctival follicles, corneal erosion, corneal infiltrate, corneal staining, edema, irritation, keratitis, keratopathy, lid disorder, lid erythema, lid margin crusting, lid retraction, lid scales, pain, photophobia.
Nonocular
Dry mouth occurred in approximately 10% of the patients.
The following adverse reactions were reported in less than 3% of the patients: abnormal coordination, asthenia, arrhythmia, asthma, chest pain, constipation, contact dermatitis, depression, dermatitis, dizziness, dry nose, dyspnea, facial edema, headache, insomnia, malaise, myalgia, nausea, nervousness, paresthesia, parosmia, peripheral edema, pharyngitis, rhinitis, somnolence, and taste perversion.
Clinical practice
The following events have been identified during post-marketing use of apraclonidine ophthalmic solution in clinical practice. Because they are reported voluntarily from a population of unknown size, estimates of frequency cannot be made. The events, which have been chosen for inclusion due to either their seriousness, frequency of reporting, possible causal connection to apraclonidine ophthalmic solution, or a combination of these factors, include: bradycardia and hypersensitivity.
Overdosage
Ingestion of apraclonidine ophthalmic solution has been reported to cause bradycardia, drowsiness, and hypothermia.
Accidental or intentional ingestion of oral clonidine has been reported to cause apnea, arrhythmias, asthenia, bradycardia, conduction defects, diminished or absent reflexes, dryness of the mouth, hypotension, hypothermia, hypoventilation, irritability, lethargy, miosis, pallor, respiratory depression, sedation or coma, seizure, somnolence, transient hypertension, and vomiting.
Treatment of an oral overdose includes supportive and symptomatic therapy; a patent airway should be maintained. Hemodialysis is of limited value since a maximum of 5% of circulating drug is removed.
Apraclonidine Dosage and Administration
One to two drops of apraclonidine ophthalmic solution should be instilled in the affected eye(s) three times daily. Since apraclonidine ophthalmic solution will be used with other ocular glaucoma therapies, an approximate 5 minute interval between instillation of each medication should be practiced to prevent washout of the previous dose. NOT FOR INJECTION INTO THE EYE. NOT FOR ORAL INGESTION.
How is Apraclonidine supplied
Apraclonidine Ophthalmic Solution, USP 0.5% as base in a sterile, isotonic, aqueous solution containing apraclonidine hydrochloride.
Supplied in a white plastic LDPE ophthalmic bottle with a natural LDPE controlled dropper tip and a purple polypropylene cap as follows:
| 5 mL | NDC 17478-716-10 |
| 10 mL | NDC 17478-716-11 |
| APRACLONIDINE OPHTHALMIC
apraclonidine hydrochloride solution/ drops |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - Akorn (117693100) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Akorn | 117696832 | MANUFACTURE(17478-716) , STERILIZE(17478-716) , ANALYSIS(17478-716) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Akorn | 117696790 | PACK(17478-716) , LABEL(17478-716) | |
More about apraclonidine ophthalmic
- Check interactions
- Compare alternatives
- Pricing & coupons
- Side effects
- Dosage information
- During pregnancy
- Drug class: ophthalmic glaucoma agents
- Breastfeeding
- En español