Lysodren Prescribing Information
Package insert / product label
Generic name: mitotane
Dosage form: tablet
Drug class: Miscellaneous antineoplastics
Medically reviewed by Drugs.com. Last updated on Jan 24, 2024.
On This Page
- Indications and Usage
- Dosage and Administration
- Dosage Forms and Strengths
- Contraindications
- Warnings and Precautions
- Adverse Reactions/Side Effects
- Drug Interactions
- Use In Specific Populations
- Overdosage
- Description
- Clinical Pharmacology
- Nonclinical Toxicology
- References
- How Supplied/Storage and Handling
- Storage and Handling
- Patient Counseling Information
- Medication Guide
Highlights of Prescribing Information
LYSODREN® (mitotane) tablets, for oral use
Initial U.S. Approval: 1970
WARNING: ADRENAL CRISIS IN THE SETTING OF SHOCK, SEVERE TRAUMA OR INFECTION
See full prescribing information for complete boxed warning.
Patients treated with LYSODREN are at increased risk for developing adrenal crisis in the setting of shock, severe trauma or infection that may lead to death.
If shock, severe trauma or infection occurs or develops, temporarily discontinue LYSODREN and administer exogenous steroids. Monitor patients closely for infections and instruct patients to contact their physician immediately if injury, infection, or any other concomitant illness occurs (2.3, 5.1).
Recent Major Changes
| BOXED WARNING | 01/2024 |
| INDICATIONS AND USAGE (1) | 01/2024 |
| DOSAGE AND ADMINISTRATION (2) | 01/2024 |
| CONTRAINDICATIONS (4) | 01/2024 |
| WARNINGS AND PRECAUTIONS (5) | 01/2024 |
Indications and Usage for Lysodren
LYSODREN is an adrenal cytotoxic agent indicated for the treatment of patients with inoperable, functional or nonfunctional, adrenocortical carcinoma (ACC). (1)
Lysodren Dosage and Administration
Dosage Forms and Strengths
Tablets: 500 mg, scored. (3)
Contraindications
None. (4)
Warnings and Precautions
- Adrenal Insufficiency and Adrenal Crisis: Temporarily withhold LYSODREN during shock, trauma, infection or adrenal insufficiency. Steroid replacement may be necessary. (5.1)
- Central Nervous System (CNS) Toxicity: Monitor behavioral and neurologic assessments and mitotane plasma levels at regular intervals. Mitotane plasma levels exceeding 20 mg/L are associated with a greater incidence of toxicity. Advise patients not to drive or operate hazardous machinery if experiencing CNS adverse reactions. (5.2)
- Ovarian Macrocysts in Premenopausal Women: Monitor pelvic ultrasound at baseline and at regular intervals. Withhold, reduce the dose, or permanently discontinue LYSODREN based on severity. (5.3)
- Hepatotoxicity: Monitor liver functions tests prior to starting LYSODREN, during dose titration and as clinically indicated. Withhold, reduce the dose or permanently discontinue based on severity. (5.4)
- Hematologic Toxicity: Monitor complete blood counts prior to starting LYSODREN, during dose titration and as clinically indicated. Withhold, reduce the dose or permanently discontinue based on severity. (5.5)
- Prolonged Bleeding Time: Prolonged bleeding time has occurred in patients treated with mitotane and this should be taken into account when surgery is considered. (5.6)
- Embryo-Fetal Toxicity: Can cause fetal harm. Advise females of reproductive potential of the potential risk to a fetus and to use effective, nonhormonal contraception. (5.8)
Adverse Reactions/Side Effects
Most common adverse reactions include: anorexia, epigastric discomfort, nausea, vomiting, diarrhea, depression, dizziness, vertigo, rash, hypercholesterolemia, hypertriglyceridemia, hypothyroidism, and decreased blood free testosterone in males. (6)
To report SUSPECTED ADVERSE REACTIONS, contact Direct Success Inc.at 1-844-597-6373 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Drug Interactions
Use In Specific Populations
- Lactation: Advise not to breastfeed. (8.2)
- Hepatic Impairment: LYSODREN is not recommended for patients with severe hepatic impairment. In patients with mild to moderate hepatic impairment, monitor mitotane plasma levels frequently and modify the dosage as needed. (8.6)
- Renal Impairment: LYSODREN is not recommended for patients with severe renal impairment. In patients with mild or moderate renal impairment, monitor mitotane plasma levels frequently and modify the dosage as needed. (8.7)
See 17 for PATIENT COUNSELING INFORMATION and Medication Guide.
Revised: 1/2024
Full Prescribing Information
WARNING: ADRENAL CRISIS IN THE SETTING OF SHOCK, SEVERE TRAUMA OR INFECTION
Patients treated with LYSODREN are at increased risk for developing adrenal crisis in the setting of shock, severe trauma or infection that may lead to death.
If shock, severe trauma or infection occurs or develops, temporarily discontinue LYSODREN and administer exogenous steroids. Monitor patients closely for infections and instruct patients to contact their physician immediately if injury, infection, or any other concomitant illness occurs [see Dosage and Administration (2.3) and Warnings and Precautions (5.1)].
1. Indications and Usage for Lysodren
LYSODREN is indicated for the treatment of patients with inoperable, functional or nonfunctional, adrenocortical carcinoma (ACC).
2. Lysodren Dosage and Administration
2.1 Recommended Evaluation and Testing Before Initiating LYSODREN
Before initiating LYSODREN, evaluate pelvic ultrasound in premenopausal women, liver functions tests and complete blood count [see Warnings and Precautions (5.3, 5.4, 5.5)].
2.2 General Precautions
LYSODREN is a hazardous drug. Advise caregivers to wear disposable gloves when handling LYSODREN tablets [see References (15) and Storage and Handling (16)].
2.3 Recommended Dosage and Administration
Recommended Dosage
- The recommended initial dose of LYSODREN is 2000 mg to 6000 mg orally, in three or four divided doses per day.
- Monitor mitotane plasma levels and increase the dose based on patient tolerance and clinical response incrementally to achieve a mitotane plasma level of 14 to 20 mg/L, or as tolerated. Consider monitoring mitotane plasma levels every 2 weeks after starting treatment and after each dose adjustment.
- The target plasma level is usually reached within a period of 3 to 5 months.
- Monitor mitotane plasma levels periodically (e.g., monthly). Severe neurotoxicity may occur with levels > 20 mg/L.
Dose Adjustments, Monitoring and Discontinuation
- In case of mitotane plasma levels above 20 mg/L without toxicities, consider reducing the dose by 50 to 75%.
- Monitor mitotane plasma levels regularly (e.g., every two months) after discontinuation of treatment. Due to the prolonged half-life, significant levels may persist for weeks after cessation of therapy.
- LYSODREN is lipophilic and accumulates in adipose tissue. Despite maintaining a constant dose, mitotane levels may suddenly increase. Monitor mitotane plasma levels even when LYSODREN has been withheld as adipose tissue may continue to release mitotane [see Clinical Pharmacology (12.3)]. Due to adipose tissue accumulation of mitotane, close monitoring of mitotane plasma levels is recommended in overweight patients and patients with recent weight loss.
Administration
- Swallow LYSODREN tablets whole; do not crush, chew or split.
- Take LYSODREN with food. Timing of the dose relative to meals must be consistent. Administration with high fat food enhances absorption [See Clinical Pharmacology Section (12.3)].
- Do not take tablets showing signs of deterioration.
- Caregivers should wear disposable gloves when handling the tablets. Avoid exposure to crushed and/or broken tablets. If contact with crushed/broken tablets occurs, wash the contaminated skin immediately and thoroughly.
- If a patient misses a dose, instruct the patient to take the next dose as scheduled.
- If a patient vomits after taking a dose, instruct the patient to take the next dose as scheduled.
2.4 Dosage Modifications for Adverse Reactions
The recommended dosage reduction for adverse reactions is to decrease the usual daily dose by 500 – 1000 mg.
Table 1 describes the dosage modifications for specific adverse reactions.
| Adverse Reaction | Severity* | Dosage Modification |
|---|---|---|
|
||
| Adrenal Crisis and Adrenal Insufficiency [see Warnings and Precautions (5.1)] | All Grades |
|
| Central Nervous System (CNS) Toxicity [see Warnings and Precautions (5.2)] | Grade 2 |
|
| Grade 3 or 4 |
|
|
| Gastrointestinal (GI) toxicity | Grade 3 or 4 |
|
| Hepatotoxicity [see Warnings and Precautions (5.4)] | Grade 3 or 4 |
|
| Hematologic Toxicity [see Warnings and Precautions (5.5)] | Grade 2 |
|
| Grade 3 or 4 |
|
|
| Other Adverse Reactions [see Adverse Reactions (6.1)] | Grade 2 |
|
| Grade 3 or 4 |
|
|
5. Warnings and Precautions
5.1 Adrenal Insufficiency and Adrenal Crisis
Adrenal Insufficiency
LYSODREN can cause adrenal insufficiency or worsen existing adrenal insufficiency in patients with adrenocortical carcinoma.
Monitor for both glucocorticoid and mineralocorticoid insufficiency and replace systemic corticosteroids accordingly. Due to increased steroid clearance and increase of steroid-binding protein, high-dose replacement therapy may be required and free cortisol and corticotropin (ACTH) should be monitored to adapt the systemic corticosteroids.
Withhold, reduce the dose, or permanently discontinue LYSODREN based on severity [see Dosage and Administration (2.4)].
Adrenal Crisis in the Setting of Shock, Severe Trauma or Infection
LYSODREN can cause adrenal suppression and adrenal crisis in the setting of shock, severe trauma or infection.
Advise patients of the signs and symptoms of adrenal suppression and to contact their healthcare provider immediately if shock, trauma, infection, or adrenal suppression occurs. Withhold LYSODREN before planned surgeries.
Temporarily withhold LYSODREN during shock, trauma, infection or adrenal suppression [see Dosage and Administration (2.4)].
Provide supportive care and administer systemic corticosteroids until recovery.
5.2 Central Nervous System Toxicity
LYSODREN can cause central nervous system toxicity, including sedation, lethargy, and vertigo [see Adverse Reactions (6.1)].
Monitor behavioral and neurologic assessments and mitotane plasma levels at regular intervals. Mitotane plasma levels exceeding 20 mg/L are associated with a greater incidence of toxicity.
In cases of cognitive dysfunction, thyroid function should be evaluated as mitotane may induce hypothyroidism.
LYSODREN can impair the ability to drive and operate machinery. Advise patients not to drive or operate hazardous machinery if they are experiencing CNS adverse reactions. Withhold, reduce the dose, or permanently discontinue LYSODREN based on severity [see Dosage and Administration (2.4)].
5.3 Ovarian Macrocysts in Premenopausal Women
LYSODREN can cause non-malignant, multiple and bilateral ovarian macrocysts in premenopausal women.
Ovarian macrocysts can be symptomatic (e.g., pelvic pain or discomfort, or menstrual irregularities) or asymptomatic.
Complications from these cysts, including adnexal torsion and hemorrhagic cyst rupture, have occurred.
Advise female patients to contact their healthcare provider immediately for gynecological symptoms such as vaginal bleeding and/or pelvic pain [see Adverse Reactions (6.1)].
Monitor pelvic imaging in premenopausal females at baseline and in regular intervals during treatment with LYSODREN.
Withhold, reduce the dose, or permanently discontinue LYSODREN based on severity [see Dosage and Administration (2.4)].
5.4 Hepatotoxicity
LYSODREN can cause hepatoxicity, including liver injury or failure.
Monitor liver function tests prior to starting treatment with LYSODREN, during dose titration, and periodically during treatment as clinically indicated.
Isolated gamma-glutamyl transferase (GGT) elevation may occur.
Withhold, reduce the dose, or permanently discontinue LYSODREN based on severity of hepatoxicity [see Dosage and Administration (2.4)].
5.5 Hematologic toxicity
LYSODREN can cause leukopenia, anemia and thrombocytopenia [see Adverse Reactions (6)]. Monitor complete blood counts including neutrophil count prior to starting treatment with LYSODREN, during dose titration, and periodically during treatment as clinically indicated. Withhold, reduce the dose, or permanently discontinue LYSODREN based on severity of cytopenia [see Dosage and Administration (2.4)].
5.6 Prolonged Bleeding Time
LYSODREN can cause platelet function disorders due to abnormal adenosine diphosphate (ADP)-induced platelet aggregation. Some patients may have a prolonged bleeding time, while others may have a normal bleeding time.
Routine in vitro bleeding time is not suitable to detect this platelet defect and to assess bleeding risk.
Perform ADP-inducted platelet aggregometry testing prior to surgery or dental procedures to determine mitotane-induced bleeding risk. For patients with prolonged bleeding time, withhold or reduce the dose of LYSODREN as clinically indicated.
5.7 Hormone binding protein
Mitotane has been shown to increase plasma levels of hormone binding proteins (e.g., sex hormone-binding globulin (SHBG) and corticosteroid-binding globulin (CBG)). This should be taken into account when interpreting the results of hormonal assays and may result in gynecomastia.
5.8 Embryo-Fetal Toxicity
LYSODREN can cause fetal harm when administered to a pregnant woman. Abnormal pregnancy outcomes, such as preterm births and early pregnancy loss, can occur in patients exposed to mitotane during pregnancy. Advise pregnant women of the potential risk to a fetus. Advise females of reproductive potential to use effective nonhormonal contraception, during treatment with LYSODREN and after discontinuation of treatment for as long as mitotane plasma levels are detectable, since LYSODREN can render some hormonal contraceptives ineffective [see Drug Interactions (7.2), Use in Specific Populations (8.1, 8.3)].
6. Adverse Reactions/Side Effects
The following clinically significant adverse reactions are described elsewhere in the labeling:
- Adrenal Insufficiency and Adrenal Crisis [see Warnings and Precautions (5.1)]
- Central Nervous System Toxicity [see Warnings and Precautions (5.2)]
- Ovarian Macrocysts in Premenopausal Women [see Warnings and Precautions (5.3)]
- Hepatotoxicity [see Warnings and Precautions (5.4)]
- Hematologic Toxicity [see Warnings and Precautions (5.5)]
- Prolonged Bleeding Time [see Warnings and Precautions (5.6)]
- Hormone Binding Protein [see Warnings and Precautions (5.7)]
- Embryo-Fetal Toxicity [see Warnings and Precautions (5.8)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Reported adverse reactions include:
- Metabolism and nutrition disorders: Anorexia
- Gastrointestinal disorders: Epigastric discomfort, nausea, vomiting, diarrhea, mucosal inflammation, dyspepsia
- Nervous system disorders: Depression, dizziness, vertigo, confusion, headache, ataxia, mental impairment, weakness, dysarthria, paresthesia, polyneuropathy, movement disorder, balance disorder, dysgeusia
- Skin and subcutaneous tissue disorders: Rash, pruritus, hypersensitivity reactions
- Blood and lymphatic system disorders: Leukopenia, anemia, thrombocytopenia, prolonged bleeding time, hematuria, hemorrhagic cystitis
- Endocrine: Growth retardation, hypothyroidism
- Eye disorders: Maculopathy, visual blurring, diplopia, lens opacity, retinopathy
- Hepatobiliary disorders: Hepatitis, elevation of liver enzymes, liver injury (hepatocellular/cholestatic/mixed)
- Reproductive system and breast disorders: Gynecomastia, hypogonadism (in males)
- Investigations: Hypercholesterolemia, hypertriglyceridemia, decreased plasma androstenedione, decreased plasma testosterone in females, increased sex hormone binding globulin in females and males, decreased blood free testosterone in males, hypouricemia
- Musculoskeletal disorders: Muscular weakness, generalized aching
- General disorders: Fever
- Renal and urinary disorders: Albuminuria/proteinuria
- Vascular disorders: Hypertension, orthostatic hypotension, flushing
- Infections: Opportunistic infection
7. Drug Interactions
7.1 Effects of Other Drugs on LYSODREN
Spironolactone
Spironolactone may block the action of mitotane. Avoid concomitant use of mitotane with spironolactone [see Clinical Pharmacology (12.3)].
7.2 Effects of LYSODREN on Other Drugs
Certain CYP3A substrates
Mitotane is a strong CYP3A inducer. Concomitant use of LYSODREN may decrease the levels of CYP3A substrates, which may reduce the activity of these substrates [see Clinical Pharmacology (12.3)].
Avoid concomitant use of LYSODREN with other CYP3A substrates, where minimal level changes may lead to serious therapeutic failures. If concomitant use cannot be avoided, modify the dosage of the CYP3A substrate in accordance with the approved product labeling.
Hormonal Contraceptives
Avoid concomitant use of LYSODREN with hormonal contraceptives [see Warnings and Precautions (5.8), Use in Specific Populations (8.3)].
Warfarin
Mitotane may induce the metabolism of warfarin, which may reduce its level and its efficacy [see Clinical Pharmacology (12.3)].
Avoid concomitant use of LYSODREN with warfarin. If concomitant use cannot be avoided, monitor INR more frequently and adjust warfarin dose as recommended in accordance with the recommendations in the warfarin Prescribing Information.
8. Use In Specific Populations
8.1 Pregnancy
Risk Summary
LYSODREN can cause fetal harm. Limited postmarketing cases report preterm births and early pregnancy loss in women treated with LYSODREN during pregnancy. Animal reproduction studies have not been conducted with mitotane.
Advise pregnant women of the potential risk to a fetus.
In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
8.2 Lactation
Risk Summary
Mitotane is excreted in human milk; however, the effect of LYSODREN on the breastfed child, or on milk production is unknown. Because of the potential for serious adverse reactions in a breastfed child, advise women not to breastfeed during treatment with LYSODREN and after discontinuation of treatment for as long as mitotane plasma levels are detectable.
8.3 Females and Males of Reproductive Potential
Pregnancy Testing
Verify the pregnancy status of females of reproductive potential prior to initiating LYSODREN [see Use in Specific Populations (8.1)].
Contraception
LYSODREN can cause fetal harm when administered to a pregnant woman [see Use in Specific Populations (8.1)].
Females
Advise females of reproductive potential to use effective nonhormonal contraception during treatment with LYSODREN and after discontinuation of therapy for as long as mitotane plasma levels are detectable [see Clinical Pharmacology (12.3)]. LYSODREN can render hormonal contraceptives ineffective [see Drug Interaction (7.2)].
8.4 Pediatric Use
Effectiveness in pediatric patients has not been established.
Based on published case reports, mitotane may negatively impact neuro-psychological development (e.g., motor and speech delay, memory impairment) in children and adolescents. In cases of cognitive dysfunction, thyroid function should be evaluated as mitotane may induce hypothyroidism. Other effects of mitotane observed in pediatric patients that are cited in medical literature or in a pharmacovigilance database include growth delay and estrogenic-like effects such as uterine bleeding, breast development in females and gynecomastia in males.
8.5 Geriatric Use
Clinical studies of LYSODREN did not include sufficient numbers of patients aged 65 years and older to determine whether they respond differently than younger patients. Other reported clinical experience has not identified differences in responses between the older and younger patients. In general, dose selection for an older patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
8.6 Hepatic Impairment
Mitotane is metabolized through the liver and mitotane plasma levels may increase if liver function is impaired.
Because of the increased risk of adverse reactions in patients with mild or moderate hepatic impairment, monitor mitotane plasma levels more frequently and modify the dosage as needed [see Dosage and Administration (2.3)]. LYSODREN is not recommended for use in patients with severe hepatic impairment [see Warnings and Precautions (5.4)].
8.7 Renal Impairment
Mitotane is eliminated through the kidney and mitotane plasma levels may increase if renal function is impaired.
Because of the increased risk of adverse reactions in patients with mild and moderate renal impairment, monitor mitotane plasma levels more frequently and modify the dosage as needed [see Dosage and Administration (2.4)]. LYSODREN is not recommended for use in patients with severe renal impairment.
10. Overdosage
LYSODREN overdosage (plasma levels are above 20 mg/L) can cause central nervous system toxicity, including sedation, lethargy, and vertigo, as well as muscular weakness and gait disturbance. Withhold LYSODREN as clinically indicated for signs or symptoms of toxicity.
LYSODREN is lipophilic and has a prolonged half-life; therefore, it may take weeks for plasma levels to decrease. LYSODREN is not likely to be dialyzable. Increase the frequency of mitotane plasma level monitoring, as clinically indicated.
11. Lysodren Description
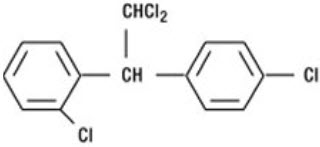
LYSODREN (mitotane) is an oral adrenal cytotoxic agent. The chemical name is (±)-1,1-dichloro-2-(o-chlorophenyl)-2-(p-chlorophenyl) ethane (also known as o,p'-DDD). The chemical structure is:
Mitotane is a white granular solid composed of clear colorless crystals. It is tasteless and has a slight pleasant aromatic odor. It is soluble in ethanol and has a molecular weight of 320.05.
Inactive ingredients in LYSODREN are: microcrystalline cellulose, polyethylene glycol 3350, silicon dioxide, and starch.
12. Lysodren - Clinical Pharmacology
12.1 Mechanism of Action
Mitotane is an adrenal cytotoxic agent with an unknown mechanism of action. Mitotane modifies the peripheral metabolism of steroids and directly suppresses the adrenal cortex. A reduction in 17-hydroxycorticosteroids in the absence of decreased corticosteroid levels and increased formation of 6-β-hydroxycortisol have been reported.
12.2 Pharmacodynamics
Mitotane exposure-response relationships and the time course of pharmacodynamic response have not been fully characterized.
12.3 Pharmacokinetics
Distribution
Mitotane is found in most tissues of the body; however, fat is the primary site of distribution.
Elimination
Following discontinuation of mitotane, the plasma terminal half-life ranges from 18 to 159 days (median 53 days).
16. How is Lysodren supplied
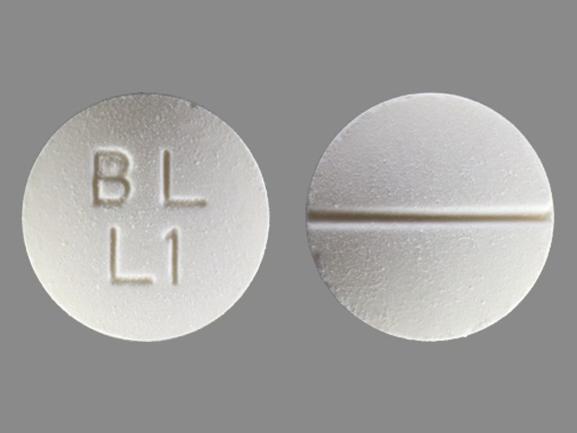
LYSODREN tablets are supplied as 500 mg white, round, biconvex, scored tablets, bisected on one side and impressed with "BL" over "L1" on the other side.
- 100 tablets per bottle: NDC 76336-080-60
Store bottles at 25°C (77°F); excursions permitted between 15°C and 30°C (59°F-86°F).
Mitotane is a hazardous drug. Follow applicable special handling and disposal procedures [see References (15)].
17. Patient Counseling Information
Advise the patient to read the FDA-approved patient labeling (Medication Guide).
Adrenal Insufficiency and Adrenal Crisis
- Advise patients of the risk of adrenal suppression that may require steroid treatment and interruption or discontinuation of LYSODREN [see Warnings and Precautions (5.1)].
- Advise patients to discontinue LYSODREN in the case of shock, severe trauma or infection and contact their healthcare provider immediately [see Warnings and Precautions (5.1)].
- Advise patients to inform their healthcare provider of any planned surgeries [see Warnings and Precautions (5.1)].
Central Nervous System Toxicity
- Advise patients to contact their healthcare provider if they experience new or worsening symptoms of central nervous system (CNS) toxicity including sedation, lethargy and vertigo [see Warnings and Precautions (5.2)].
- Instruct patients not to drive or operate hazardous machinery if they are experiencing CNS adverse reactions [see Warnings and Precautions (5.2)].
Ovarian Macrocysts in Premenopausal Women
- Advise premenopausal women that their healthcare provider will monitor them with routine imaging and to contact their healthcare provider if they experience gynecological symptoms such as vaginal bleeding and/or pelvic pain [see Warnings and Precautions (5.3)].
Hepatotoxicity
- Advise patients that their healthcare provider will monitor them with laboratory tests to monitor liver function and to immediately contact their healthcare provider to report signs and symptoms of hepatotoxicity [see Warnings and Precautions (5.4)].
Hematologic Toxicity
- Advise patients to immediately contact their healthcare provider for fever, other signs of infection, unusual bruising, bleeding, tiredness or pallor [see Warnings and Precautions (5.5)].
Prolonged Bleeding Time
- Advise patients of the possibility of prolonged bleeding time while taking LYSODREN and of the need to withhold LYSODREN if surgery or dental procedures are needed [see Warnings and Precautions (5.6)].
- Advise patients to inform their healthcare provider of any planned surgeries or dental procedures [see Warnings and Precautions (5.6)].
- Advise patients to contact their healthcare provider for bruising or bleeding [see Warnings and Precautions (5.6)].
Embryo-Fetal Toxicity
- Advise females of reproductive potential of the potential risk to a fetus and to inform their healthcare provider of a known or suspected pregnancy [see Warnings and Precautions (5.8) and Use in Specific Populations (8.1)].
Females of Reproductive Potential
- Advise females of reproductive potential to use effective nonhormonal contraception during treatment with LYSODREN and after discontinuation of treatment for as long as mitotane plasma levels are detectable, since LYSODREN can render some hormonal contraceptives ineffective [see Drug Interactions (7.2), Use in Specific Populations (8.3)].
Lactation
- Advise females not to breastfeed during treatment with LYSODREN [see Use in Specific Populations (8.2)].
Drug Interactions
- Advise patients and their caregivers to inform their healthcare providers of all concomitant medications, herbal and dietary supplements [see Drug Interactions (7.1, 7.2)].
Address medical inquiries to:
Direct Success Inc.
1710 Hwy 34
Farmingdale, NJ 07727
1-844-597-6373
Fax: 1-855-674-6767
Manufactured by:
Latina Pharma S.p.A.
Via Murillo, 7
04013 Sermoneta (Latina)
Italy
For: HRA Pharma Rare Diseases
| MEDICATION GUIDE LYSODREN® (LY-SO-DREN) (mitotane) tablets, for oral use |
||||||
|---|---|---|---|---|---|---|
| This Medication Guide has been approved by the U.S. Food and Drug Administration. | Issued: 01/2024 | |||||
| What is the most important information I should know about LYSODREN? | ||||||
| LYSODREN can cause serious side effects including: | ||||||
Adrenal Insufficiency and Adrenal Crisis.
|
||||||
| Your healthcare provider will check your levels of corticosteroid hormones during treatment and may give you corticosteroid medicine if you develop adrenal gland problems. Tell your healthcare provider right away if you develop any signs or symptoms of adrenal gland problems, including: | ||||||
|
|
|
||||
| See "What are the possible side effects of LYSODREN?" for more information about side effects. | ||||||
| What is LYSODREN? | ||||||
| LYSODREN is a prescription medicine used to treat people with cancer of the adrenal glands (adrenocortical carcinoma) that is functional (when the adrenal glands make more corticosteroid hormone than normal) or nonfunctional (when the adrenal glands make less corticosteroid hormone than normal) and the cancer cannot be removed by surgery. | ||||||
| Effectiveness in pediatric patients has not been established. | ||||||
Before taking LYSODREN, tell your healthcare provider about all of your medical conditions, including if you:
|
||||||
| Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. Taking LYSODREN with certain other medicines may affect the way LYSODREN and the other medicines work and may increase your risk of side effects. | ||||||
| Especially tell your healthcare provider if you take: | ||||||
|
|
|
||||
| Ask your healthcare provider about any other medicine that may not be listed above. | ||||||
| Know the medicines you take. Keep a list of them to show your healthcare provider and pharmacist when you get a new medicine. | ||||||
How should I take LYSODREN?
|
||||||
| What should I avoid while taking LYSODREN? | ||||||
| Do not drive or operate machinery until you know how LYSODREN affects you. LYSODREN may cause sleepiness, decreased energy, and dizziness, which may affect your ability to drive and operate machinery. | ||||||
| What are the possible side effects of LYSODREN? | ||||||
| LYSODREN can cause serious side effects including: | ||||||
|
||||||
|
|
|||||
| Your healthcare provider may test your blood to make sure your thyroid is producing enough thyroid hormone and to check the mitotane level if you develop any of these signs and symptoms during treatment. | ||||||
|
||||||
|
|
|||||
|
||||||
|
|
|||||
|
||||||
|
|
|||||
|
||||||
| Your healthcare provider will do blood tests before you start treatment with LYSODREN, during your treatment, and after you stop treatment with LYSODREN to check mitotane levels in your body and to check for side effects. | ||||||
| Your healthcare provider may change your dose, temporarily stop, or permanently stop treatment with LYSODREN if you develop certain side effects. | ||||||
| The most common side effects of LYSODREN include: | ||||||
|
|
|||||
| These are not all of the possible side effects of LYSODREN. | ||||||
| Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. | ||||||
| You may also report side effects to Direct Success Inc. at 1-844-597-6373. | ||||||
| How should I store LYSODREN? | ||||||
| Store LYSODREN at 77°F (25°C). | ||||||
| Keep LYSODREN and all medicines out of the reach of children. | ||||||
| General information about the safe and effective use of LYSODREN. | ||||||
| Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use LYSODREN for a condition for which it was not prescribed. Do not give LYSODREN to other people, even if they have the same symptoms that you have. It may harm them. You can ask your pharmacist or healthcare provider for information about LYSODREN that is written for health professionals. | ||||||
| What are the ingredients in LYSODREN? | ||||||
| Active ingredient: mitotane | ||||||
| Inactive ingredients: microcrystalline cellulose, polyethylene glycol 3350, silicon dioxide, and starch. | ||||||
| Manufactured by: Latina Pharma S.p.A., Via Murillo, 7, 04013 Sermoneta (Latina), Italy; Manufactured for HRA Pharma Rare Diseases. | ||||||
| For more information, go to www.lysodren.com or call 1-844-597-6373. | ||||||
| LYSODREN
mitotane tablet |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - HRA Pharma Rare Diseases (571682231) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Corden Pharma Latina S.p.A. | 339062883 | MANUFACTURE(76336-080) , ANALYSIS(76336-080) , LABEL(76336-080) , PACK(76336-080) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| ISP Chemicals LLC | 078413681 | API MANUFACTURE(76336-080) | |
More about Lysodren (mitotane)
- Check interactions
- Compare alternatives
- Pricing & coupons
- Drug images
- Side effects
- Dosage information
- During pregnancy
- Drug class: miscellaneous antineoplastics
- En español