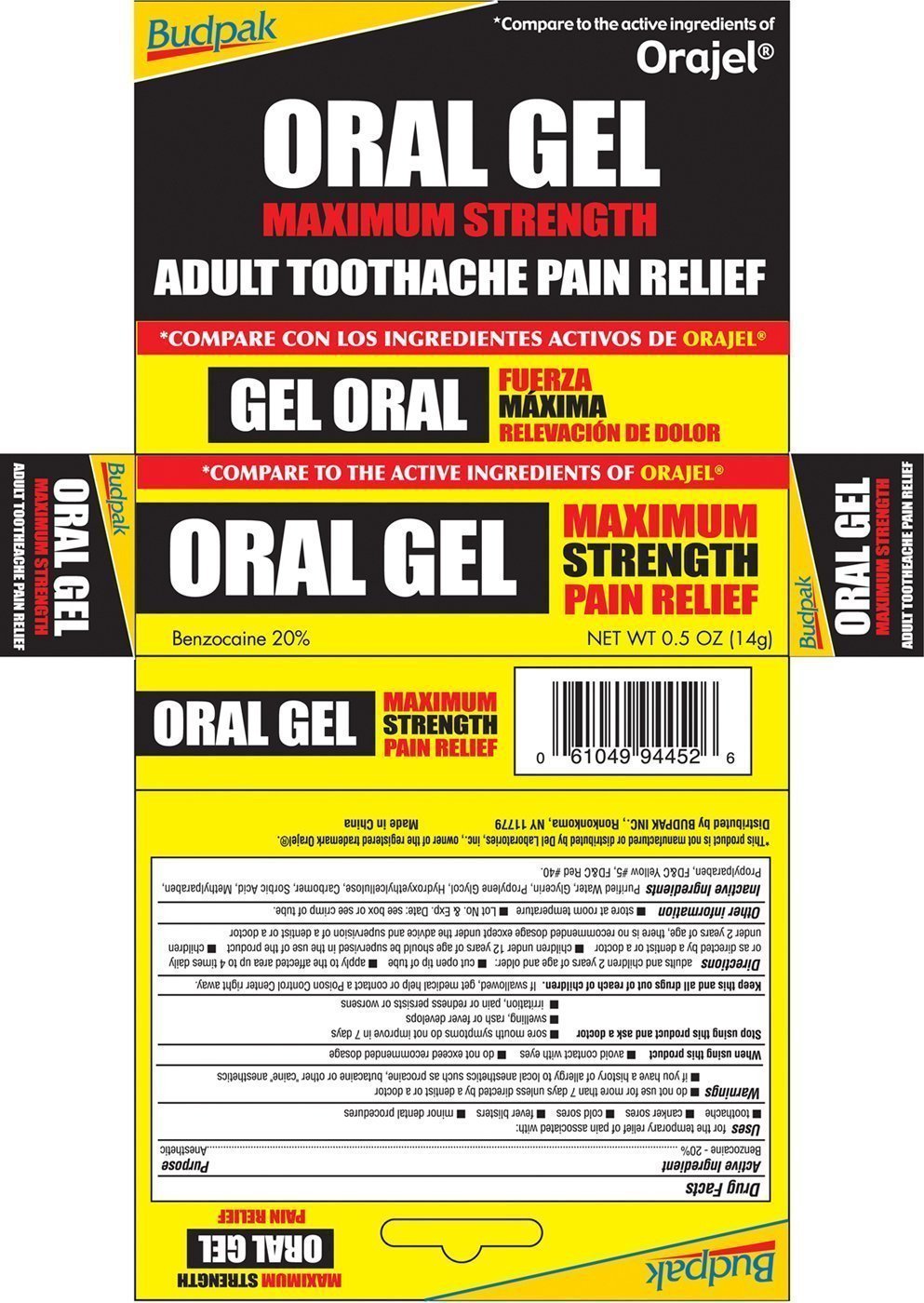
Oral Gel Prescribing Information
Package insert / product label
Generic name: benzocaine
Dosage form: gel
Drug class: Topical anesthetics
Medically reviewed by Drugs.com. Last updated on Mar 25, 2024.
On This Page
Uses for the temporary relief of pain associated with:
- toothache
- canker sores
- cold sores
- fever blisters
- minor dental procedures
Warnings
- do not use for more than 7 days unless directed by a dentist or a doctor
- if you have a history of allergy to local anesthetics such as procaine, butacaine or other "caine" anesthetics
Stop using and ask a doctor
- sore mouth symptoms do not improve in 7 days
- swelling, rash or fever develops
- irritation, pain or redness persists or worsens
Keep this and all drugs out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away
Directions adults and children 2 years of age and older:
- cut open tip of tube
- apply to the affected area up to 4 times daily or as directed by a dentist or doctor
- children under 12 years of age should be supervised in the use of the product
- children under 2 years of age, there is no recommended dosage except under the advice and supervision of a dentist or a doctor
| ORAL GEL
MAXIMUM STRENGTH
benzocaine gel |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Budpak Inc. (183224849) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Ausmetics Daily Chemicals (Guangzhou) Co. Ltd. | 529836561 | manufacture | |
More about benzocaine topical
- Check interactions
- Compare alternatives
- Reviews (39)
- Latest FDA alerts (5)
- Side effects
- Dosage information
- During pregnancy
- Drug class: topical anesthetics
- Breastfeeding
Patient resources
Professional resources
Other brands
Benzo-Jel, Topical Anesthetic Dental Gel, BeeGentle, Denti-Care Denti-Freeze, ... +2 more