Pentothal Prescribing Information
Package insert / product label
Generic name: thiopental sodium
Dosage form: injection, powder, for solution
Drug class: General anesthetics
Medically reviewed by Drugs.com. Last updated on Mar 25, 2024.
On This Page
Pentothal Description
Pentothal (Thiopental Sodium for Injection) is a thiobarbiturate, the sulfur analogue of sodium pentobarbital.
The drug is prepared as a sterile powder and after reconstitution with an appropriate diluent is administered by the intravenous route.
Pentothal is chemically designated sodium 5-ethyl-5-(1-methylbutyl)-2-thiobarbiturate and has the following structural formula:
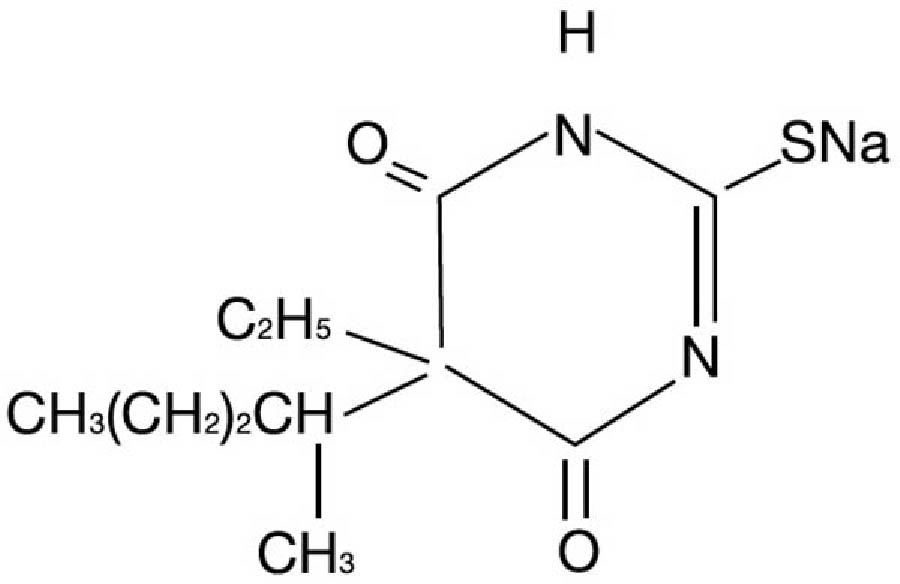
The drug is a yellowish, hygroscopic powder, stabilized with anhydrous sodium carbonate as a buffer (60 mg/g of thiopental sodium).
Pentothal - Clinical Pharmacology
Pentothal (Thiopental Sodium for Injection) is an ultrashort-acting depressant of the central nervous system that induces hypnosis and anesthesia, but not analgesia. It produces hypnosis within 30 to 40 seconds of intravenous injection. Recovery after a small dose is rapid, with some somnolence and retrograde amnesia. Repeated intravenous doses lead to prolonged anesthesia because fatty tissues act as a reservoir; they accumulate Pentothal in concentrations 6 to 12 times greater than the plasma concentration, and then release the drug slowly to cause prolonged anesthesia.
The half-life of the elimination phase after a single intravenous dose is three to eight hours.
The distribution and fate of Pentothal (as with other barbiturates) is influenced chiefly by its lipid solubility (partition coefficient), protein binding and extent of ionization. Pentothal has a partition coefficient of 580.
Approximately 80% of the drug in the blood is bound to plasma protein. Pentothal is largely degraded in the liver and to a smaller extent in other tissues, especially the kidney and brain. It has a pKa of 7.4.
Concentration in spinal fluid is slightly less than in the plasma.
Biotransformation products of thiopental are pharmacologically inactive and mostly excreted in the urine.
Related/similar drugs
gabapentin, clonazepam, lamotrigine, pregabalin, topiramate, diazepam, lidocaine
Indications and Usage for Pentothal
Pentothal (Thiopental Sodium for Injection) is indicated (1) as the sole anesthetic agent for brief (15 minute) procedures, (2) for induction of anesthesia prior to administration of other anesthetic agents, (3) to supplement regional anesthesia, (4) to provide hypnosis during balanced anesthesia with other agents for analgesia or muscle relaxation, (5) for the control of convulsive states during or following inhalation anesthesia, local anesthesia, or other causes, (6) in neurosurgical patients with increased intracranial pressure, if adequate ventilation is provided, and (7) for narcoanalysis and narcosynthesis in psychiatric disorders.
Contraindications
Absolute Contraindications:
(1) Absence of suitable veins for intravenous administration, (2) hypersensitivity (allergy) to barbiturates and (3) variegate porphyria (South African) or acute intermittent porphyria.
Relative Contraindications:
(1) Severe cardiovascular disease, (2) hypotension or shock, (3) conditions in which the hypnotic effect may be prolonged or potentiated — excessive premedication, Addison’s disease, hepatic or renal dysfunction, myxedema, increased blood urea, severe anemia, asthma, myasthenia gravis, and (4) status asthmaticus.
Warnings
KEEP RESUSCITATIVE AND ENDOTRACHEAL INTUBATION EQUIPMENT AND OXYGEN READILY AVAILABLE. MAINTAIN PATENCY OF THE AIRWAY AT ALL TIMES.
This drug should be administered only by persons qualified in the use of intravenous anesthetics.
Avoid extravasation or intra-arterial injection.
Precautions
Observe aseptic precautions at all times in preparation and handling of Pentothal (Thiopental Sodium for Injection) solutions.
If used in conditions involving relative contraindications, reduce dosage and administer slowly.
Care should be taken in administering the drug to patients with advanced cardiac disease, increased intracranial pressure, ophthalmoplegia plus, asthma, myasthenia gravis and endocrine insufficiency (pituitary, thyroid, adrenal, pancreas).
Drug interactions: The following drug interactions have been reported with thiopental.
|
Drug |
Effect |
|
Probenecid |
Prolonged action of thiopental |
|
Diazoxide |
Hypotension |
|
Zimelidine |
Thiopental antagonism |
|
Opioid analgesics |
Decreased antinociceptive action |
|
Aminophylline |
Thiopental antagonism |
|
Midazolam |
Synergism |
Nursing Mothers: Thiopental sodium readily crosses the placental barrier and small amounts may appear in the milk of nursing mothers following administration of large doses.
Pregnancy Category C. Animal reproduction studies have not been conducted with Pentothal. It is also not known whether Pentothal can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Pentothal should be given to a pregnant woman only if clearly needed.
Adverse Reactions/Side Effects
Adverse reactions include respiratory depression, myocardial depression, cardiac arrhythmias, prolonged somnolence and recovery, sneezing, coughing, bronchospasm, laryngospasm and shivering. Anaphylactic and anaphylactoid reactions to Pentothal (Thiopental Sodium for Injection) have been reported. Symptoms, e.g., urticaria, bronchospasm, vasodilation and edema should be managed by conventional means.
Rarely, immune hemolytic anemia with renal failure and radial nerve palsy have been reported.
Overdosage
Overdosage may occur from too rapid or repeated injections. Too rapid injection may be followed by an alarming fall in blood pressure even to shock levels. Apnea, occasional laryngospasm, coughing and other respiratory difficulties with excessive or too rapid injections may occur. In the event of suspected or apparent overdosage, the drug should be discontinued, a patent airway established (intubate if necessary) or maintained, and oxygen should be administered, with assisted ventilation if necessary. The lethal dose of barbiturates varies and cannot be stated with certainty. Lethal blood levels may be as low as 1 mg/100 mL for short-acting barbiturates; less if other depressant drugs or alcohol are also present.
MANAGEMENT OF OVERDOSAGE
It is generally agreed that respiratory depression or arrest due to unusual sensitivity to thiopental sodium or overdosage is easily managed if there is no concomitant respiratory obstruction. If the airway is patent, any method of ventilating the lungs (that prevents hypoxia) should be successful in maintaining other vital functions. Since depression of respiratory activity is one of the characteristic actions of the drug, it is important to observe respiration closely.
Should laryngeal spasm occur, it may be relieved by one of the usual methods, such as the use of a relaxant drug or positive pressure oxygen. Endotracheal intubation may be indicated in difficult cases.
Pentothal Dosage and Administration
Pentothal is administered by the intravenous route only. Individual response to the drug is so varied that there can be no fixed dosage. The drug should be titrated against patient requirements as governed by age, sex and body weight. Younger patients require relatively larger doses than middle-aged and elderly persons; the latter metabolize the drug more slowly. Pre-puberty requirements are the same for both sexes, but adult females require less than adult males. Dose is usually proportional to body weight and obese patients require a larger dose than relatively lean persons of the same weight.
Premedication
Premedication usually consists of atropine or scopolamine to suppress vagal reflexes and inhibit secretions. In addition, a barbiturate or an opiate is often given. Sodium pentobarbital injection (Nembutal®) is suggested because it provides a preliminary indication of how the patient will react to barbiturate anesthesia. Ideally, the peak effect of these medications should be reached shortly before the time of induction.
Test Dose
It is advisable to inject a small “test” dose of 25 to 75 mg (1 to 3 mL of a 2.5% solution) of Pentothal (Thiopental Sodium for Injection) to assess tolerance or unusual sensitivity to Pentothal, and pausing to observe patient reaction for at least 60 seconds. If unexpectedly deep anesthesia develops or if respiratory depression occurs, consider these possibilities: (1) the patient may be unusually sensitive to Pentothal, (2) the solution may be more concentrated than had been assumed, or (3) the patient may have received too much premedication.
Use in Anesthesia
Moderately slow induction can usually be accomplished in the “average” adult by injection of 50 to 75 mg (2 to 3 mL of a 2.5% solution) at intervals of 20 to 40 seconds, depending on the reaction of the patient. Once anesthesia is established, additional injections of 25 to 50 mg can be given whenever the patient moves.
Slow injection is recommended to minimize respiratory depression and the possibility of overdosage. The smallest dose consistent with attaining the surgical objective is the desired goal. Momentary apnea following each injection is typical, and progressive decrease in the amplitude of respiration appears with increasing dosage. Pulse remains normal or increases slightly and returns to normal. Blood pressure usually falls slightly but returns toward normal. Muscles usually relax about 30 seconds after unconsciousness is attained, but this may be masked if a skeletal muscle relaxant is used. The tone of jaw muscles is a fairly reliable index. The pupils may dilate but later contract; sensitivity to light is not usually lost until a level of anesthesia deep enough to permit surgery is attained. Nystagmus and divergent strabismus are characteristic during early stages, but at the level of surgical anesthesia, the eyes are central and fixed. Corneal and conjunctival reflexes disappear during surgical anesthesia.
When Pentothal (Thiopental Sodium for Injection) is used for induction in balanced anesthesia with a skeletal muscle relaxant and an inhalation agent, the total dose of Pentothal can be estimated and then injected in two to four fractional doses. With this technique, brief periods of apnea may occur which may require assisted or controlled pulmonary ventilation. As an initial dose, 210 to 280 mg (3 to 4 mg/kg) of Pentothal is usually required for rapid induction in the average adult (70 kg).
When Pentothal (Thiopental Sodium for Injection) is used as the sole anesthetic agent, the desired level of anesthesia can be maintained by injection of small repeated doses as needed or by using a continuous intravenous drip in a 0.2% or 0.4% concentration. (Sterile water should not be used as the diluent in these concentrations, since hemolysis will occur.) With continuous drip, the depth of anesthesia is controlled by adjusting the rate of infusion.
Use in Convulsive States
For the control of convulsive states following anesthesia (inhalation or local) or other causes, 75 to 125 mg (3 to 5 mL of a 2.5% solution) should be given as soon as possible after the convulsion begins. Convulsions following the use of a local anesthetic may require 125 to 250 mg of Pentothal given over a ten minute period. If the convulsion is caused by a local anesthetic, the required dose of Pentothal will depend upon the amount of local anesthetic given and its convulsant properties.
Use in Neurosurgical Patients with Increased Intracranial Pressure
In neurosurgical patients, intermittent bolus injections of 1.5 to 3.5 mg/kg of body weight may be given to reduce intraoperative elevations of intracranial pressure, if adequate ventilation is provided.
Use in Psychiatric Disorders
For narcoanalysis and narcosynthesis in psychiatric disorders, premedication with an anticholinergic agent may precede administration of Pentothal. After a test dose, Pentothal (Thiopental Sodium for Injection) is injected at a slow rate of 100 mg/min (4 mL/min of a 2.5% solution) with the patient counting backwards from 100. Shortly after counting becomes confused but before actual sleep is produced, the injection is discontinued. Allow the patient to return to a semidrowsy state where conversation is coherent. Alternatively, Pentothal may be administered by rapid I.V. drip using a 0.2% concentration in 5% dextrose and water. At this concentration, the rate of administration should not exceed 50 mL/min.
MANAGEMENT OF SOME COMPLICATIONS
Respiratory depression (hypoventilation, apnea), which may result from either unusual responsiveness to Pentothal or overdosage, is managed as stated above. Pentothal should be considered to have the same potential for producing respiratory depression as an inhalation agent, and patency of the airway must be protected at all times.
Laryngospasm may occur with light Pentothal narcosis at intubation, or in the absence of intubation if foreign matter or secretions in the respiratory tract create irritation. Laryngeal and bronchial vagal reflexes can be suppressed, and secretions minimized by giving atropine or scopolamine premedication and a barbiturate or opiate. Use of a skeletal muscle relaxant or positive pressure oxygen will usually relieve laryngospasm. Tracheostomy may be indicated in difficult cases.
Myocardial depression, proportional to the amount of drug in direct contact with the heart, can occur and may cause hypotension, particularly in patients with an unhealthy myocardium. Arrhythmias may appear if PCO2 is elevated, but they are uncommon with adequate ventilation. Management of myocardial depression is the same as for overdosage. Pentothal (Thiopental Sodium for Injection) does not sensitize the heart to epinephrine or other sympathomimetic amines.
Extravascular infiltration should be avoided. Care should be taken to insure that the needle is within the lumen of the vein before injection of Pentothal. Extravascular injection may cause chemical irritation of the tissues varying from slight tenderness to venospasm, extensive necrosis and sloughing. This is due primarily to the high alkaline pH (10 to 11) of clinical concentrations of the drug. If extravasation occurs, the local irritant effects can be reduced by injection of 1% procaine locally to relieve pain and enhance vasodilatation. Local application of heat also may help to increase local circulation and removal of the infiltrate.
Intra-arterial injection can occur inadvertently, especially if an aberrant superficial artery is present at the medial aspect of the antecubital fossa. The area selected for intravenous injection of the drug should be palpated for detection of an underlying pulsating vessel. Accidental intra-arterial injection can cause arteriospasm and severe pain along the course of the artery with blanching of the arm and fingers. Appropriate corrective measures should be instituted promptly to avoid possible development of gangrene. Any patient complaint of pain warrants stopping the injection. Methods suggested for dealing with this complication vary with the severity of symptoms. The following have been suggested:
- Dilute the injected Pentothal (Thiopental Sodium for Injection) by removing the tourniquet and any restrictive garments.
- Leave the needle in place, if possible.
- Inject the artery with a dilute solution of papaverine, 40 to 80 mg, or 10 mL of 1% procaine, to inhibit smooth muscle spasm.
- If necessary, perform sympathetic block of the brachial plexus and/or stellate ganglion to relieve pain and assist in opening collateral circulation. Papaverine can be injected into the subclavian artery, if desired.
- Unless otherwise contraindicated, institute immediate heparinization to prevent thrombus formation.
- Consider local infiltration of an alpha-adrenergic blocking agent such as phentolamine into the vasospastic area.
- Provide additional symptomatic treatment as required.
Shivering after Pentothal anesthesia, manifested by twitching face muscles and occasional progression to tremors of the arms, head, shoulder and body, is a thermal reaction due to increased sensitivity to cold. Shivering appears if the room environment is cold and if a large ventilatory heat loss has been sustained with balanced inhalation anesthesia employing nitrous oxide. Treatment consists of warming the patient with blankets, maintaining room temperature near 22° C (72° F), and administration of chlorpromazine or methylphenidate.
PREPARATION OF SOLUTIONS
Pentothal (Thiopental Sodium for Injection) is supplied as a yellowish, hygroscopic powder. Solutions should be prepared aseptically with the following diluent: Sterile Water for Injection, USP. Reconstitute 500 mg Pentothal with 20 mL Sterile Water for Injection, USP. Reconstitute 1 g Pentothal with 20 mL Sterile Water for Injection, USP. Clinical concentrations used for intermittent intravenous administration vary between 2.0% and 5.0%. A 2.0% or 2.5% solution is most commonly used. A 3.4% concentration in sterile water for injection is isotonic; concentrations less than 2.0% in this diluent are not used because they cause hemolysis. For continuous intravenous drip administration, concentrations of 0.2% or 0.4% are used. Solutions may be prepared by adding Pentothal to 5% Dextrose Injection, USP, 0.9% Sodium Chloride Injection, USP or Normosol®-R pH 7.4.
Since Pentothal contains no added bacteriostatic agent, extreme care in preparation and handling should be exercised at all times to prevent the introduction of microbial contaminants. Solutions should be freshly prepared and used promptly. Sterilization by heating should not be attempted.
COMPATIBILITY
Any solution of Pentothal (Thiopental Sodium for Injection) with a visible precipitate should not be administered. The stability of Pentothal solutions depends upon several factors, including the diluent, temperature of storage and the amount of carbon dioxide from room air that gains access to the solution. Any factor or condition which tends to lower pH (increase acidity) of Pentothal solutions will increase the likelihood of precipitation of thiopental acid. Such factors include the use of diluents which are too acidic and the absorption of carbon dioxide which can combine with water to form carbonic acid.
Solutions of succinylcholine, tubocurarine or other drugs which have an acid pH should not be mixed with Pentothal solutions. The most stable solutions are those reconstituted in water or isotonic saline, kept under refrigeration and tightly stoppered. The presence or absence of a visible precipitate offers a practical guide to the physical compatibility of prepared solutions of Pentothal.
| CALCULATIONS FOR VARIOUS CONCENTRATIONS | |||
| Concentration Desired | Amount to Use | ||
| Percent | mg/mL | Pentothal g | Diluent mL |
| 0.2 | 2 | 1 |
500 |
| 0.4 | 4 | {1 | 250 |
| 2 | 500 | ||
| 2.0 | 20 | {5 | 250 |
| 10 | 500 | ||
| 2.5 | 25 | {1 | 40 |
| 5 | 200 | ||
| 5 | 50 | {1 | 20 |
| 5 | 100 | ||
Reconstituted solutions of Pentothal (Thiopental Sodium for Injection) should be inspected visually for particulate matter and discoloration, whenever solution and container permit.
Warnings
Intravenous administration of Sterile Water for Injection, USP without a solute may result in hemolysis.
Use aseptic technique for preparing Pentothal solutions during withdrawal from reconstituted single -use containers.
Administer only clear reconstituted solutions.
Use within 24 hours after reconstitution. Discard unused portions.
Precautions
Do not use unless solution is clear and container is undamaged.
Inspect reconstituted (mixed) solutions of Pentothal (Thiopental Sodium for Injection) for clarity and freedom from precipitation or discoloration prior to administration. Use reconstituted solution only if it is clear, free from precipitate and not discolored.
Pregnancy Category C. Animal reproduction studies have not been conducted with sterile water for injection or sodium chloride injection. It is also not known whether sterile water or sodium chloride injection containing additives can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Sterile water for injection or sodium chloride injection with additives should be given to a pregnant woman only if clearly needed.
Adverse Reactions/Side Effects
Reactions which may occur because of the diluent, technique of preparation or mixing, or administration of reconstituted solutions of Pentothal include febrile response or infection at the site of injection, venous thrombosis or phlebitis extending from the site of injection and extravasation.
If an adverse reaction does occur, discontinue the injection, evaluate the patient, institute appropriate therapeutic countermeasures and save the remainder of unused solution (or the used container or syringe) for examination if deemed necessary.
Pentothal Dosage and Administration
Pentothal solutions should be administered only by intravenous injection and by individuals experienced in the conduct of intravenous anesthesia.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. See PRECAUTIONS.
Store at 20 to 25°C (68 to 77°F). [See USP Controlled Room Temperature.]
Keep reconstituted solution in a cool place.
|
NDC No. |
Pentothal |
Pentothal
Container |
Diluent (mL)* |
Theoretical
Reconstituted Conc. |
|
|
|
|
|
|
|
0409-3158-10 |
500 mg |
Vial |
W (20) |
2.5% (25 mg/mL) |
|
0409-6431-10 |
1 g |
Vial |
W (20) |
5% (50 mg/mL) |
|
|
|
|
|
|
Revised: April, 2010
Made in Italy K156946A
Hospira, Inc., Lake Forest, IL 60045 USA
| THIOPENTAL SODIUM
thiopental sodium injection, powder, for solution |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| THIOPENTAL SODIUM
thiopental sodium injection, powder, for solution |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Hospira, Inc. (141588017) |
More about Pentothal (thiopental)
- Check interactions
- Compare alternatives
- Reviews (3)
- Side effects
- Dosage information
- During pregnancy
- Drug class: general anesthetics
- Breastfeeding

